The product details are as follows:

Endotoxin
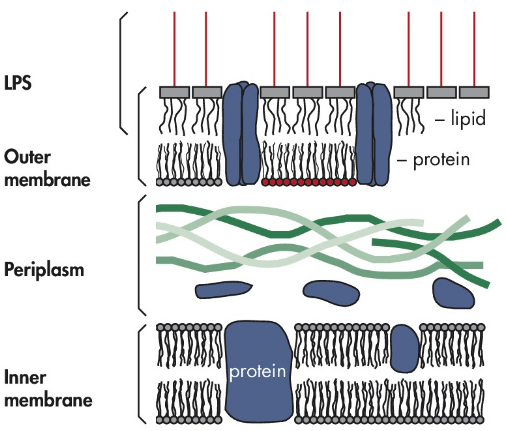
Endotoxin, also known as lipopolysaccharide or LPS, is a key component of the outer membrane of Gram-negative bacteria such as *E. coli*. The lipid portion of this membrane is primarily made up of endotoxin molecules. A single *E. coli* cell contains approximately 2 million LPS molecules, each composed of a hydrophobic lipid A, a sugar residue, and a negatively charged phosphate group. This unique structure gives endotoxin both hydrophobic and hydrophilic properties, allowing it to interact with various biological molecules. During bacterial growth or death, a small amount of endotoxin is released into the environment. When lysing bacterial cells for plasmid preparation, endotoxin is released from the outer membrane into the lysate. Endotoxin significantly affects biological applications, particularly in DNA transfection processes. It strongly reduces the efficiency of transfection in primary and sensitive cultured cells. For gene therapy, using endotoxin-free plasmid DNA is crucial because endotoxin can trigger fever, endotoxic shock, and activate the complement cascade in humans and animals. Additionally, endotoxin interferes with immune responses by activating macrophages and B cells, leading to the release of inflammatory mediators like IL-1 and prostaglandins. To ensure accurate results, it's essential that plastic products, media, serum, and plasmid DNA are free from LPS contamination. Endotoxin assays typically rely on the clotting reaction between endotoxin and clotting proteins found in limulus amebocyte lysate (LAL). More advanced photometric methods, such as the Kinetic-QCL Test, now allow for more sensitive detection using synthetic substrates. LPS contamination is usually measured in endotoxin units (EU), where 1 ng of LPS corresponds to 1–10 EU. To remove endotoxins, commercial kits are widely used. Polymyxin B is commonly immobilized on agarose microspheres to specifically bind and remove endotoxins through electrostatic, ionic, and hydrophilic interactions. Some available products include: - **PurKine Endotoxin Removal Kit (Polycide B)** – 1ml×5/3ml×5, Product No. KTP21400 - **PurKine Endotoxin Removal Resin** – 5ml/100ml, Product No. BMR21400 - **PurKine Endotoxin Clear Prepacked Column** – 1ml×5/3ml×5, Product No. BMC21400 During the removal process, it’s important to use endotoxin-free plastic and glassware. Recontamination can occur if non-pyrogen-free materials are used after initial purification. Glassware can strongly bind endotoxins, making them difficult to remove completely. Standard autoclaving has limited effect on endotoxin levels, while heating at 180°C overnight may help destroy residual endotoxins. Always use reagents that are endotoxin-free to avoid compromising the final product.Ultraviolet Climate Resistance Test Chamber
Ultraviolet Climate Resistance Test Chamber,Uv Intensity Test Chamber,Sun Resistant Test Chamber,Sunlight Exposure Test Chamber
Wuxi Juxingyao Trading Co., Ltd , https://www.juxingyao.com