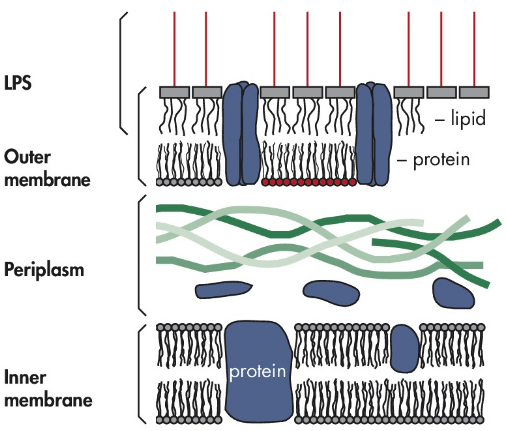
Endotoxin
Endotoxin, also known as lipopolysaccharide (LPS), is a key component of the outer membrane of Gram-negative bacteria such as *E. coli*. It consists of a hydrophobic lipid A region, a core polysaccharide, and an O-antigen. Each LPS molecule contains hydrophobic, hydrophilic, and charged regions, allowing it to interact with various biological molecules. During bacterial growth or lysis, endotoxins are released into the environment. This makes them a critical concern in laboratory settings, especially when isolating plasmid DNA.
Effect of endotoxin on biological applications
Endotoxins can severely impact cell transfection efficiency, particularly in primary and sensitive cultured cells. High levels of endotoxin can lead to reduced transfection success and even cell death. In gene therapy, using endotoxin-free plasmid DNA is essential, as endotoxins can trigger fever, septic shock, and immune activation in humans and animals. They also interfere with immune cell function by inducing the production of inflammatory mediators like IL-1 and prostaglandins. To ensure accurate results, all materials—such as plastics, media, serum, and DNA—must be free from LPS contamination.
Endotoxin assay
Traditional endotoxin detection methods rely on the clotting reaction between LPS and clotting proteins in limulus amebocyte lysate (LAL). Modern photometric assays, such as the Kinetic-QCL Test, offer greater sensitivity by using synthetic substrates. LPS contamination is usually measured in endotoxin units (EU), where 1 ng of LPS corresponds to approximately 1–10 EU. These tests are vital for quality control in biopharmaceutical and research environments.
Endotoxin removal
Commercial endotoxin removal kits are widely used today. Polymyxin B is commonly immobilized on agarose beads to bind and remove endotoxins through electrostatic, ionic, and hydrophobic interactions. Key products include:
| PurKine Endotoxin Removal Kit (Polycide B) | 1ml×5/3ml×5 | KTP21400 | Details |
| PurKine Endotoxin Removal Resin | 5ml/100ml | BMR21400 | Details |
| PurKine Endotoxin Clear Prepacked Column | 1ml×5/3ml×5 | BMC21400 | Details |
When removing endotoxins, it's crucial to use endotoxin-free labware and reagents. Recontamination after initial removal can occur if non-pyrogen-free materials are used. Glassware should be cleaned thoroughly, and heating at 180°C overnight can help eliminate residual endotoxins. Always ensure that all reagents and equipment used in DNA purification are endotoxin-free to maintain sample integrity and experimental accuracy.
Ozone Aging Test Chamber,Oxidation Degree Testing Test Machine,Ozone Resistance Testing Test Box,Rubber Aging Test Machine
Wuxi Juxingyao Trading Co., Ltd , https://www.juxingyao.com